High-tech tampons and CRISPR therapies
The words on everyone’s lips the past few weeks? Gene therapy.
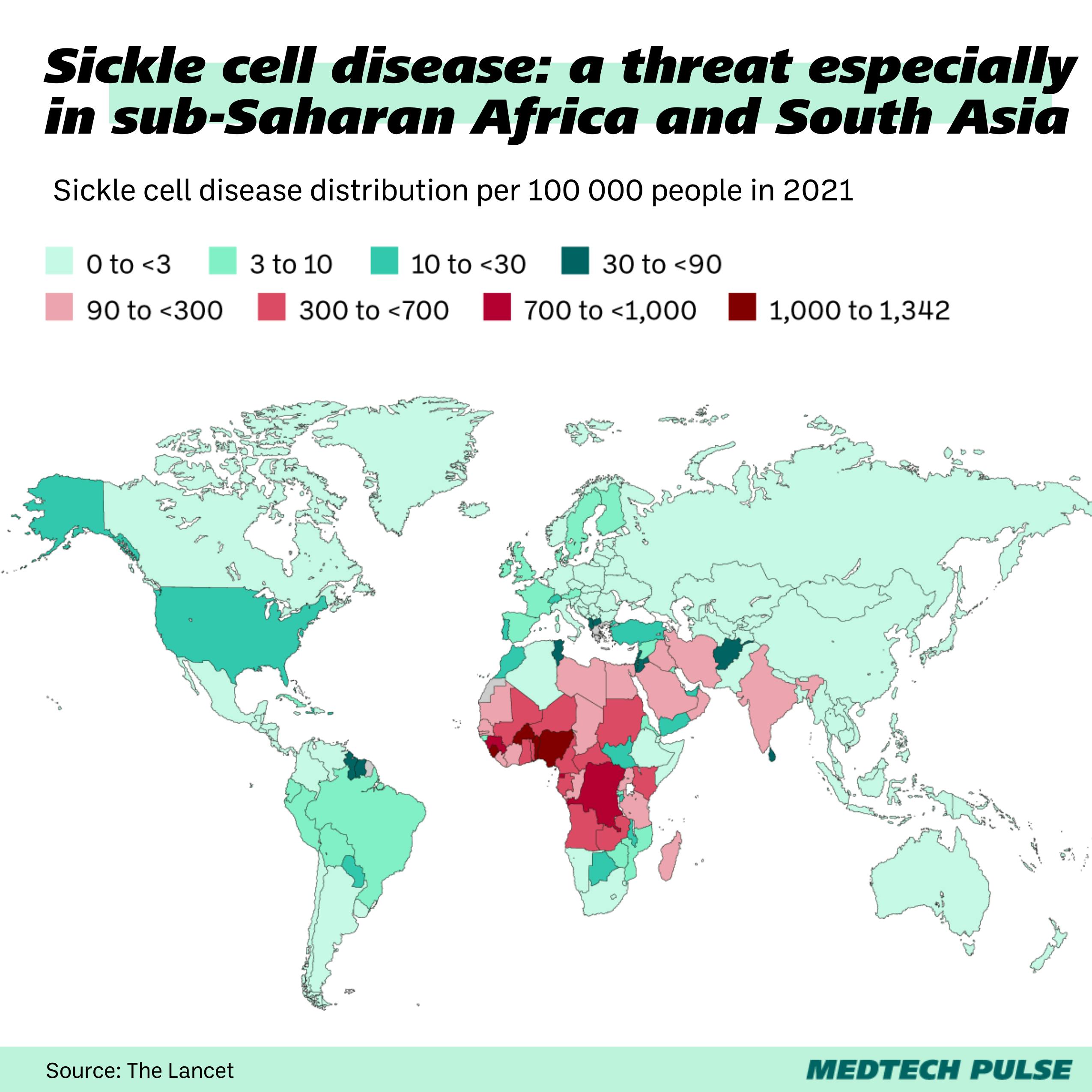
Last month, the U.K. became the first country to approve the first CRISPR-based gene-editing therapy. The medicine, Casgevy, treats sickle cell disease (SCD) and beta-thalassemia. The U.S. approved the therapy this month, and Europe looks poised to join soon too.
These approvals are historic achievements.
It’s been just a few years since Emmanuelle Charpentier and Jennifer Doudna won the Nobel Prize in Chemistry for pioneering the use of CRISPR-Cas9 for gene editing. Medical research has been taking advantage of the technology for years. Now, patients are going to benefit in the most direct way yet.
SCD and thalassemia cause patients excruciating pain without many options for relief. This treatment is going to give many people around the world a long-overdue path toward a healthier future.
But Casgevy is not a magic pill. Taking it is no walk in the park.
Patients who decide to go through the gene-editing therapy must commit to weeks in the hospital and the many unpleasant side effects of the preparatory chemotherapy. It’s understandable that many may continue to be hesitant toward treatment despite how debilitating their conditions are. After all, it’s staying with the devil they know, versus the devil they don’t.
Plus, with the treatment being so ground-breaking and new, the current pricing is astronomical. Countries and health systems are already worried about projected affordability issues.
This is why, even when we celebrate a new milestone in medical innovation, we must not forget to keep our eyes trained on the new opportunities and challenges it presents.
With this win, we’re faced with new puzzles for improving the patient experience, comfort, and adherence. In a similar vein, in this week’s Insights, we’re celebrating STI-screening tampon technology and incredibly portable flatpack field hospitals—innovations that aren’t entirely new like Casgevy is. Rather, these products are iterations on existing technology which make them better.
One of the hardest parts of working in our industry is resisting the urge to make our celebrations declarations of total victory.
We’re seeing this with how medicine is grappling with the evolving roles of medical AI—even calling for a new Hippocratic oath. We’re also seeing it with how new findings about the short-term efficacy of GLP-1s tempt some to call the drugs an end to obesity. We see it with cancer screening and treatments that, while effective, put patients through discomfort and don’t work as well for all bodies.
For SCD and thalassemia patients, trading one painful experience for another with Casgevy likely doesn’t feel like an unequivocal triumph.
For patients like them, we must keep pushing our creativity and striving for better. Until we’ve completely eradicated a disease, no innovation can ever be “good enough.”
Let’s stay focused on the work and pushing forward for that healthy, pain-free future for all.
