Long-awaited pulse oximeter skin tone testing guidance released by the FDA
Finally, a life-saving medical device will work well for all patients. Or, at least, that’s the ultimate goal of the newly-released testing guidance.
The latest: Decades after studies first showed pulse oximeters work less effectively on people with darker skin tones, the FDA has finally released updated guidance for how to test these devices to improve accuracy for all patients.
- The Covid-19 pandemic prompted this reevaluation, when pulse oximeters became even more of a crucial tool than they already were. Back in 2021, the FDA issued a safety alert after studies showed that Black patients receiving supplemental oxygen were more likely to have lower oxygen levels than the oximeter readings suggested.
- The FDA took up this inquiry to revise the standing 2013 guidance in 2022, finally acknowledging the gravity of this inequitable device efficacy.
Melanin and light: The reason why patients with darker skin tones often receive inaccurate pulse oximetry readings is both simple and complicated.
- The simple explanation: Melanin partially absorbs light that hits skin, including the light pulse oximeters use for readings. The more melanin, the less light passes through the skin, the more potentially inaccurate the readings.
- However, for years, manufacturers have largely denied that these inaccuracies were significant and could impact patient care. The studies didn’t make it into medical training, leaving even well-intentioned providers largely in the dark.
Revamping pulse oximetry studies: The new guidance won’t result in new, better pulse oximeters immediately, but it’s an important first step.
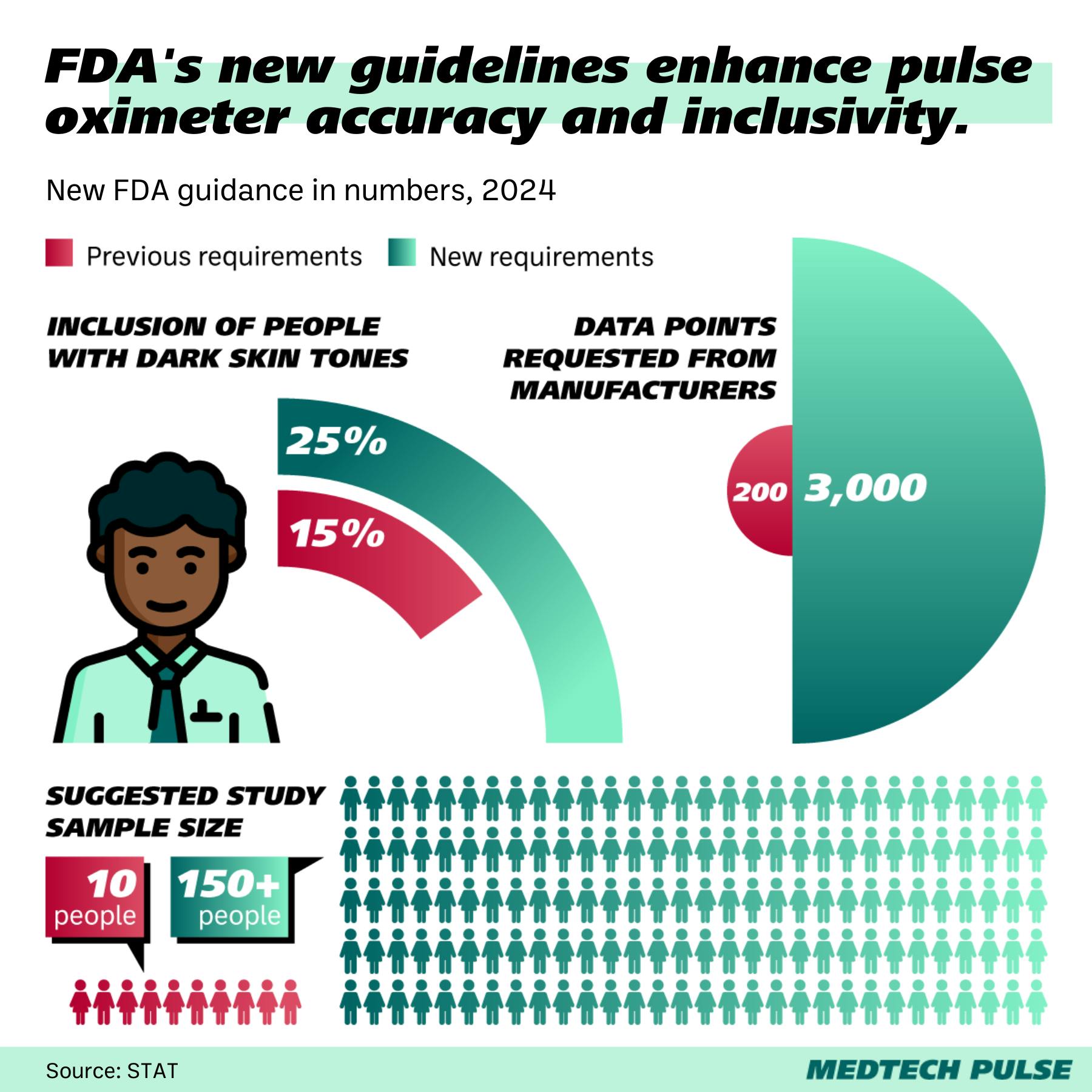
- The guidance is grounded in the Monk Skin Tone Scale, an open-source ten-shade skin tone scale developed in partnership with Google for better skin color representation, as well as the individual typology angle (ITA), which is based on spectrophotometric measurements.
- Per the new guidance, when designing pulse oximetry studies, at least 25% of subjects need to be in each of three groups: those with light, medium, and dark skin tones. Plus, half the participants in the dark-skin tone group must include subjects with very dark skin.
As with many initiatives to make medtech tools work better for all, innovation begins with research and data diversity. This years-long process of overhauling FDA guidance goes to show that sometimes even deciding what data diversity looks like can be a challenge.
We salute the researchers and patient advocates who have worked to make this step a reality, leading to a world where all patients can safely go under anesthesia and receive supplemental oxygen, trusting that the tools monitoring their blood oxygen work as they should.